Preventive vaccines against infectious diseases
1.The prompt approval of the mRNA-based vaccine for the prevention of infectious disease
The history of vaccine for the prevention of infectious disease can be traced back to Edward Jenner’s discovery that cowpox can be used as a smallpox vaccine at the end of the 18th century. Since then, vaccines have prevented many infectious diseases, greatly contributing to public health. There was a major innovation changing the classical preventive infectious vaccine during the COVID-19 pandemic which started in 2020.
The COVID-19 mRNA vaccine is a new type of vaccine that induces immunity to viruses within the body after the administration of mRNA which includes the genetic information of viruses. It may be still fresh in your memory that the mRNA vaccine was developed and approved only one year after identification of the virus.
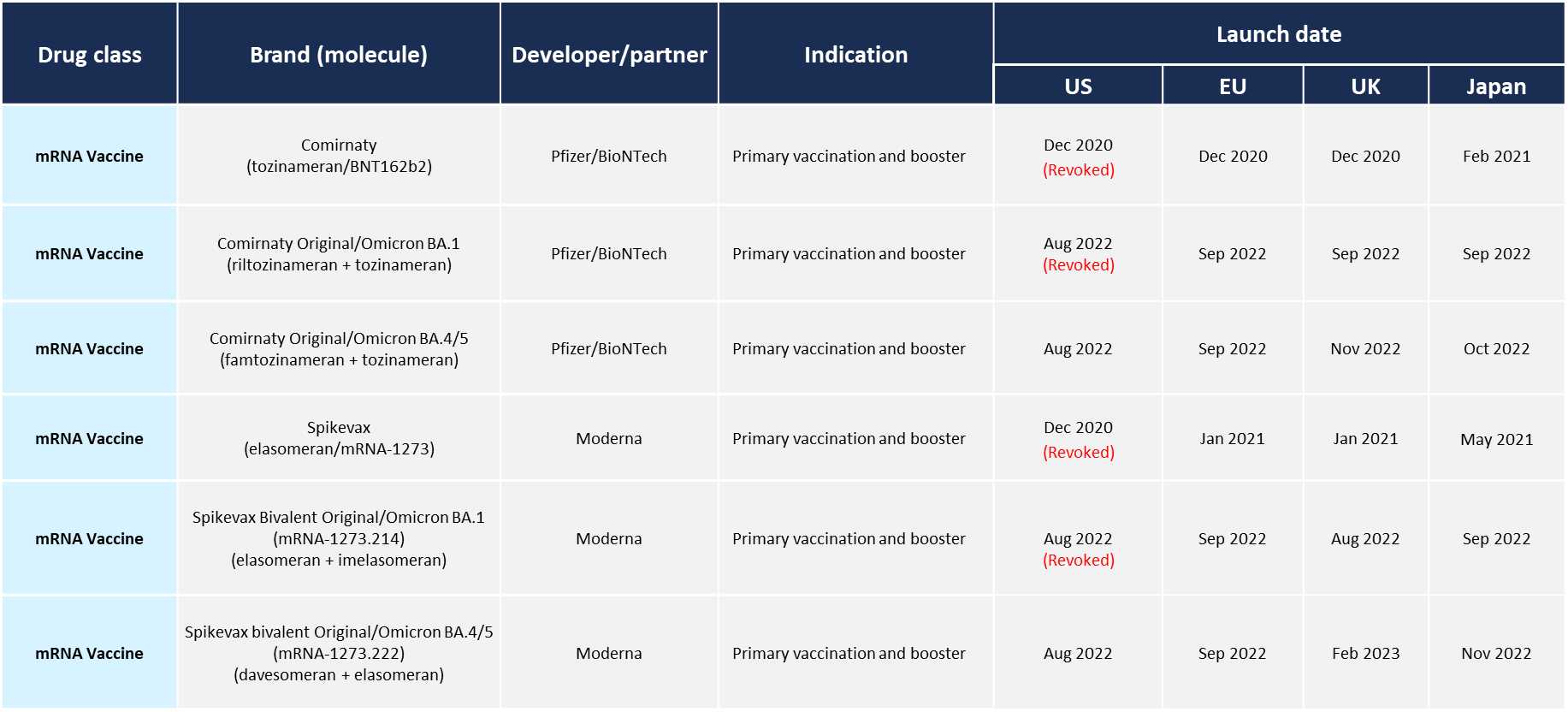
There are six COVID-19 mRNA vaccine products manufactured by BioNTech/Pfizer or Moderna that have been approved in Japan, the USA and five countries in Europe. Currently, the BioNTech/Pfizer COMIRNATY and Moderna Spikevax monovalent vaccines for the Omicron BA4/5 variant are available (Table 1). In Japan, there are other approved products. DS-5670 is an mRNA vaccine targeting the receptor binding domain of spike protein from Daiichi Sankyo and ARCT-154 is a replicon mRNA vaccine encoding the full-length spike protein) from Meiji Seika Pharma/Arcturus Therapeutics.
Table 1: COVID-19 mRNA vaccines approved in Japan, the USA and five countries in Europe*1
2.Trends in the infectious disease prevention vaccine market and development
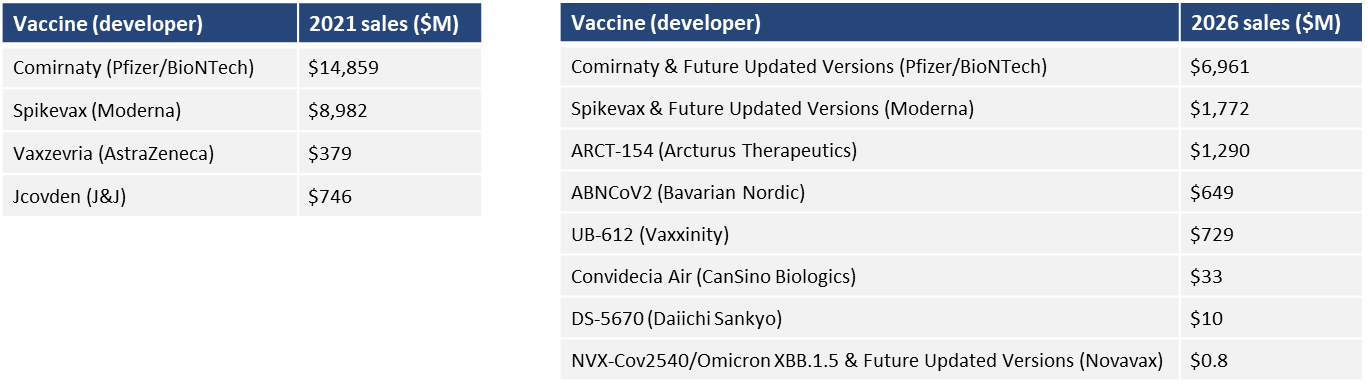
In 2021, the total sales of COMIRNATY and Spikevax in Japan, the USA and Europe was approximately 2.6 billion dollars (3.6 trillion yen at 140 yen to the dollar), and the total sales of the four products in 2026 is forecast to be approximately one billion dollars (1.4 trillion yen). The market is expected to stay at a certain scale for two years. Many COVID-19-related vaccines are still in late-phase development. About 50% of those are mRNA vaccine products. This includes pan-coronavirus vaccines and vaccines that offer effective long-term protection from SARS-CoV-2 variants.
Table 2: COVID-19 vaccines in 2021 and 2026*1
Nearly 1000 vaccines for the infectious disease were under development around the world at the beginning of 2023*2. Classical inactivated or attenuated vaccines are 23% of the vaccines being developed. This is followed by recombinant protein vaccines (20%), gene-based vaccines (e.g., DNA and mRNA; 18%), viral vector vaccines (14%), and conjugate vaccines (11%). Recombinant protein vaccines are dominant because of their safety, stability and manufacturability. Phase 1 studies are ongoing for over 100 products. DNA/mRNA vaccines are considered to be appropriate for vaccination against the viruses that mutate very frequently because of the high flexibility of manufacturing. In total, over 130 products are now under development for the inoculation of people against viruses as such as SARS-CoV-2, influenza, and HIV.
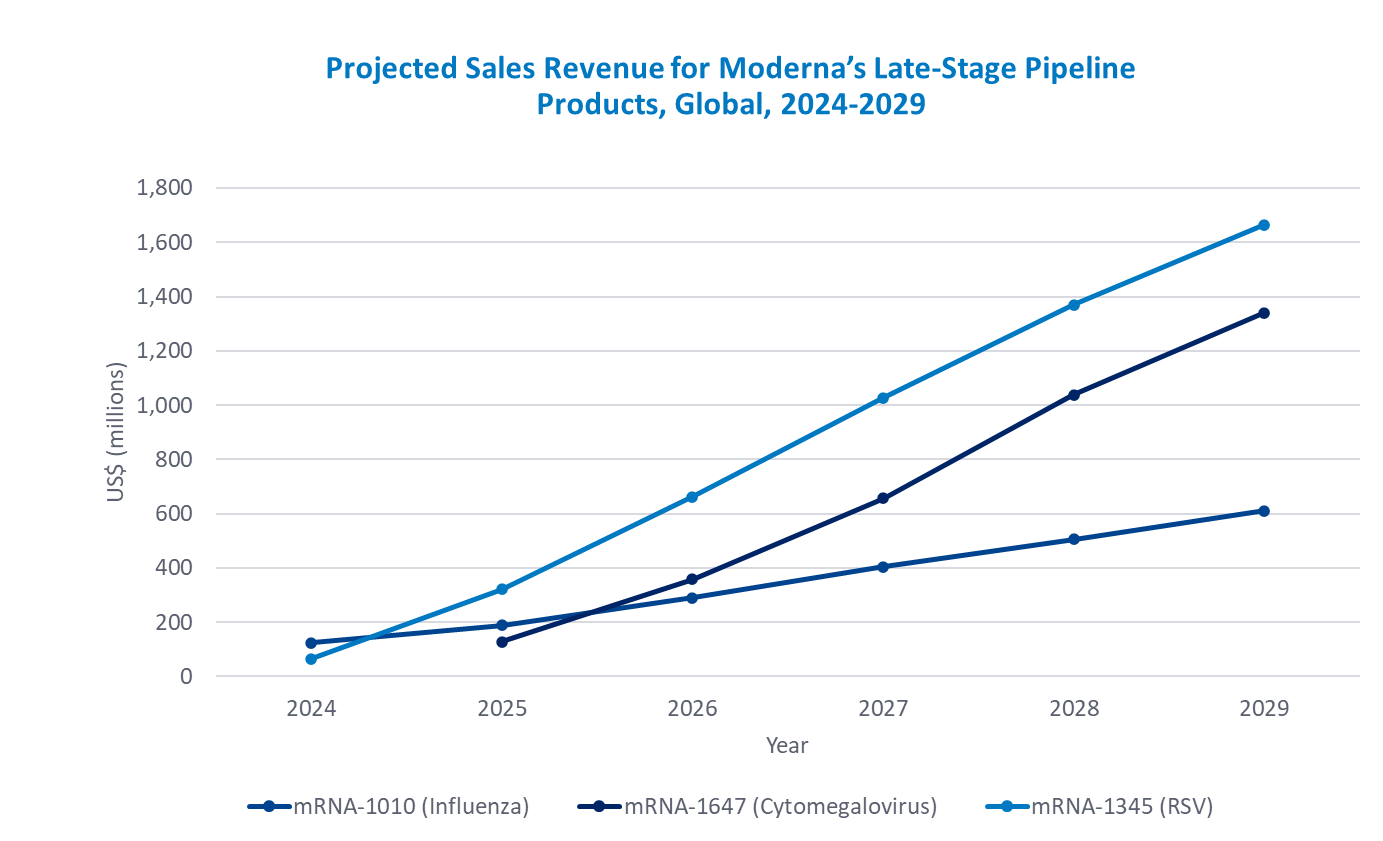
In areas other than COVID-19, there are ongoing phase 3 studies for two seasonal influenza mRNA vaccines (BioNTech’s BNT-161 and Moderna’s mRNA-1010), a bivalent SARS-CoV-2 and seasonal influenza vaccine (Moderna’s mRNA-1083), a CMV vaccine (Moderna’s mRNA-1647), and an RSV vaccine (Moderna’s mRNA-1345). This reveals Modern’s position as the leader in the development of mRNA vaccines.3 There are 25 products, including 13 influenza products (including two bivalent vaccines for SARS-CoV-2 as well), five herpes virus vaccines, and two monkeypox virus vaccines in mRNA vaccine pipeline that currently have phase 2 studies ongoing. According to the market forecast for the three Moderna mRNA vaccines in late-phase development, the peak market size for the CMV vaccine (mRNA-1647) is estimated to be 85 billion yen. For the RSV vaccine (mRNA-1345) it is estimated to be 230 billion yen, and for the influenza vaccine (mRNA-1010), 190 billion yen. When all of them are approved, the total market size will reach 500 billion yen*3.
3.SCARDA, a domestic R&D initiative preparing for the next pandemic
The final section describes Japan’s response to the COVID-19 pandemic.
In light of the National Strategy for Strengthening the Vaccine Development and Production System approved by the Cabinet on June 1, 2021, the Strategic Center of Biomedical Advanced Vaccine Research and Development for Preparedness and Response (SCARDA) was established on March 22, 2022. SCARDA collects and analyzes a wide range of information about vaccine development even during times when there isn’t an ongoing pandemic to provide strategic research expense funding to prepare emergencies caused by infections. It carries out projects to build the world-leading research and development bases for vaccine/novel modality R&D projects and vaccine development and also serves as an organization for management and general coordination in normal and emergency conditions.
Participating collaborator for the Vaccine/New Modality Research and Development Project, one of SCARDA’s projects adopted in October 2022, NANO MRNA will take charge of a nonclinical study and the following Phase 1 study in and after 2024.
The Establishment of a PureCap-method-based Domestic Production Structure of High-purity mRNA Vaccine and Clinical Development of Safe mRNA Vaccines
R&D representative: Dr. Satoshi Uchida, Crafton Biotechnology Co., Ltd.
*1 Global Data: COVID-19 Vaccines: Opportunity Assessment and Forecast, 2023
*2 Nature reviews drug discovery 22, 867–868, 2023
*3 Global Data: mRNA Vaccines in Infectious Diseases Market Overview, 2023
