Cancer vaccine
1. Neoantigens and mRNA vaccines
In the cancerization process, there is an accumulation of many mutated somatic genes. The products (proteins) of these mutated genes are degraded by proteasomes within the cytoplasm, similar to the degradation of non-mutated products, bonded to MHC Class 1 molecules, and presented on the surface of cells as an antigenic peptide–MHC complex. Among the antigenic peptides, the peptides with sequences derived from genetic mutations have immunogenicity as neoantigens and become the target of cytotoxic CD8-positive T cells since they are not subject to immunological tolerance because they are different from the innately non-mutated peptides similar to non-self-antigens such as bacteria and viruses.
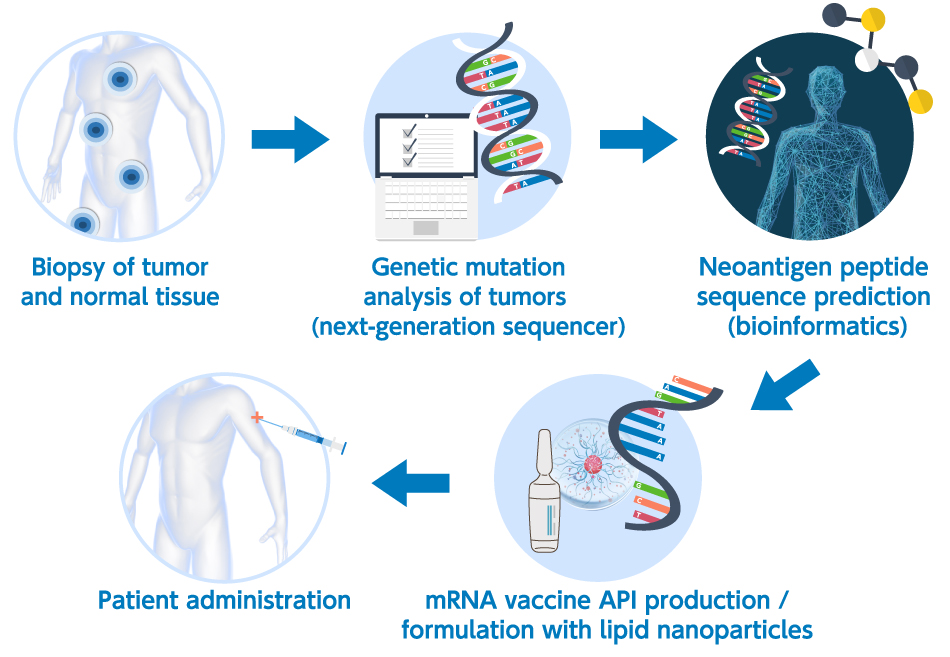
Next-generation sequencing (NGS) analysis has facilitated human genome mapping, enabled the full exon sequencing of the tumors in each patient, and permitted the prediction of the peptides that bond with MHC Class I molecules (epitopes) using a predictive algorithm for MHC bonding based on the results of the sequencing analysis. The advent of NGS and the rapid advancement of bioinformatics have resulted in the in silico prediction of the neoantigens in each patient. The ultimate in personalized therapy is mRNA neoantigen vaccines combined with in silico neoantigen prediction (Figure 1 below shows a general neoantigen mRNA vaccine manufacturing scheme).
2. Therapeutic vaccines for cancer based on mRNA
Two mRNA biotech giants, Moderna and BioNTech, are actively promoting the clinical development of neoantigen vaccines for cancer.
Moderna is developing mRNA-4157, a LNP-based drug using mRNA that encodes an average of 34 neoantigens. BioNTech is advancing the development of autogene cevumeran (BNT122), a polyplex-based drug consisting of mRNA that encodes up to 20 neoantigens. For both of these investigational drugs, the first-in-human Phase I study was performed to examine the safety in single-agent administration and it was confirmed that CD8-positive T cells were induced for the neoantigens derived from several mRNAs, and POC has been established in single-agent administration.
- For mRNA-4157, a Phase III study is underway after recurrence-free survival time was found to be significantly longer than with single-agent treatment using pembrolizumab, an immune checkpoint inhibitor, in a randomized Phase II study in which pembrolizumab was concomitantly used in adjuvant treatment following surgical operations for melanoma.
- The clinical development of BNT122 confirmed the trend toward the remarkable prolongation of recurrence-free survival time was only observed in pancreatic cancer patients showing induction of neoantigen-specific CD8-positive CTL compared with the group treated with the immune checkpoint inhibitor atezolizumab, chemotherapy as standard care, and BNT122 as a combination therapy in adjuvant treatment following surgery.
These results have already resulted in innovation in cancer immunotherapy. Clinical trial results draw attention prior to drug approval review.
Table 1: Neoantigen RNA vaccines under clinical development
