Genome Editing
1. World’s first genome editing therapy approved in 2023
On November 16, 2023, the Medicines and Healthcare products Regulatory Agency (MHRA) of the U. K. has given the world’s first approval to a therapeutic drug based on genome editing. The therapeutic drug is CASGEVY [generic name: exagamglogene autotemcel (exa-cel)], jointly developed by Vertex Pharmaceuticals and CRISPR Therapeutics, which is being indicated for sickle cell disease and beta thalassemia. These diseases can lead to severe anemia or other conditions because they hinder the production of normal red blood cells due to genetic mutation.
CASGEVY involves the collection of hematopoietic stem cells from the bone marrow of patients, and the extracorporeal use of CRISPR/Cas9 enzyme and guide RNA for genome editing at specific sites of the enhancer of the BCL11A gene, followed by the infusion back to the body. Humans have finally acquired a new modality, genome editing. However, there is still an issue of convenience since CASGEVY is an ex vivo therapy that requires genome editing outside the body.
2. Development ongoing for genome editing therapies based on mRNA
NTLA-2001 (Intellia Therapeutics) is a therapy that involves intravenous injection of more convenient CRISPR/Cas9 enzyme in patients in the form of mRNA. On the NEJMC, one of the most authoritative medical journals in the world, there was a report on results of a Phase 1 study involving patients with familial transthyretin amyloidosis (ATTR).
ATTR will occur when transthyretin loses its stability and turns into amyloid, which accumulates in the heart and/or the nervous system. Symptoms of the disease include such neural symptoms as central nervous system disorder, sensorimotor neuropathy and autonomic disorder; renal disorder; myocardial disorder; and vitreous opacity.
NLAT-2001
It knocks out the transthyretin genes of hepatic cells using LNP’s property of gathering in hepatic cells.
When NTLA-2001 was administered to six patients with the disease in the Phase 1 study, the blood transthyretin level dropped dose-dependently without serious adverse reactions. A high dose group was then added. This confirmed a decrease in blood transthyretin levels of at least 90% and safety in a group treated with 1 mg/kg. Intellia Therapeutics announced on October 28, 2023 that it had reached an agreement with the FDA on the performance of a Phase 3 study in patients with cardiac amyloidosis.
The company is also developing NTLA-2002, the same type of genome editing therapy. The drug is to be indicated for hereditary angioedema (HAE). They made a presentation on the development at ACAA in 2022.
NLAT-2002
In the Phase 1/2 study in progress, there were no serious adverse reactions in any of the ten enrolled patients. When the highest dose 75 mg was administered, it was confirmed that the plasma kallikrein protein level fell 90% or more, and the frequency of HAE attacks was clearly reduced. The manufacturer announced that it would proceed to a Phase 2 study.
3. Increased attention on a new genome editing technology
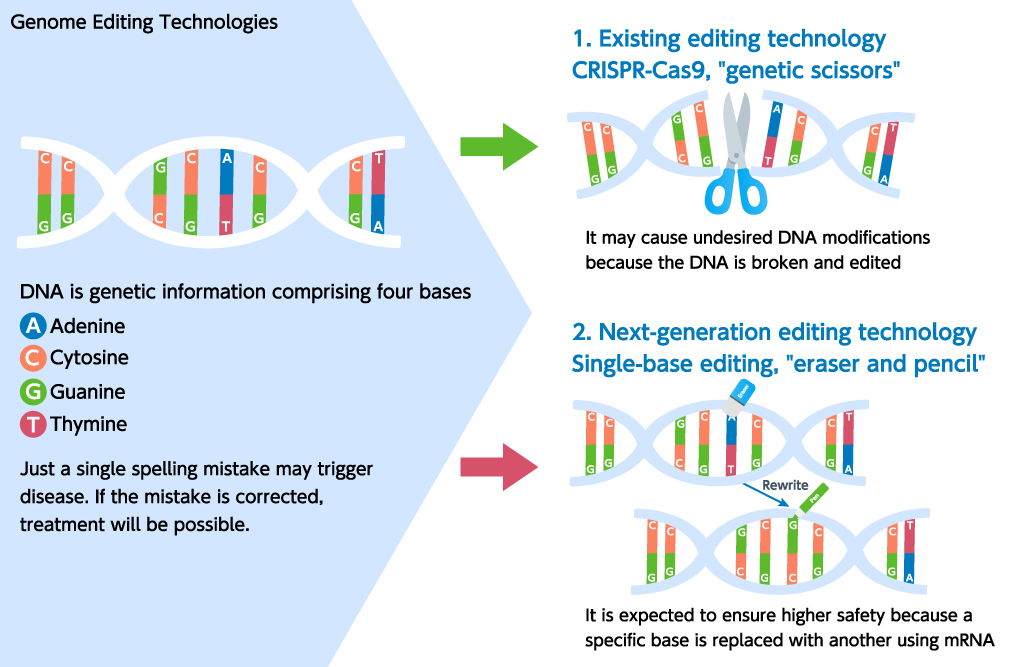
Tumorigenicity and cytotoxicity are concerns associated with CRISPR/Cas9 because of accompanying DNA double-strand breaks. A new genome editing technology is therefore attracting attention as an application of single-base editing. For the this reason, new technology “rewiting” consists inactivation of nuclease and addition of deaminase activity. If adenosine deaminase is used, it will enable the “rewriting” of adenine to guanine.
Applying this principle, Verve Therapeutics developed VERVE-101, a single-base editing therapy aimed at turning off the PSCK9 gene and achieving lower blood LDL-cholesterol (LDL-C).
VERVE-101
They have already disclosed the initial results of the Phase 1 study: PSCK9 and LDL-C dropped without serious adverse reactions up to 0.6 mg/kg. Clinical research is ongoing.
Investigational genome editing therapy based on mRNA
